
Botox & Fillers

Enhance Your Natural Beauty with Botox Cosmetic & Fillers in Tampa
Over time, skin becomes less elastic; repeated frowning creates lines and wrinkles between the brows (also known as glabellar lines). BOTOX® Cosmetic is the only FDA-approved prescription treatment for moderate to severe frown lines between the brows in people ages 18 to 65. The American Society for Aesthetic Plastic Surgery ranked BOTOX® Cosmetic as the most popular physician-administered cosmetic procedure in the United States for the 4th year in a row; almost 3.3 million BOTOX® Cosmetic procedures were performed in 2005 alone.
BOTOX® Cosmetic is a purified protein that smooths the muscles by blocking nerve impulses. With a few tiny injections, BOTOX® Cosmetic blocks the release of acetylcholine, the chemical that causes muscles to contract. With less movement, the skin surface gradually smooths out and the lines begin to fade.
The BOTOX® Cosmetic procedure consists of a few tiny injections and takes only 10 minutes. No anesthesia is required; however Dr. Tomlinson may numb the area with a cold pack or anesthetic cream prior to injecting. There may be minimal and brief discomfort, along with slight temporary bruising at the injection sight which can easily be covered up with make-up. There is no down-time.
Results are typically seen in days and may continue to improve during the first week after treatment. Results may vary. With proper unit dosing, the effects of BOTOX® Cosmetic can last up to four months. 89% of men and women surveyed rated the improvement in their appearance of brow frown lines as moderate to better after one month.
To ensure the best treatment outcome, Dr. Tomlinson should inject 20 units in the glabellar region between the brows - ensure you receive the proper dosage for best results! Dosage amount is not based on the number of syringes used - ask Dr. Tomlinson exactly how many units you are receiving.
BOTOX® can be used for therapeutic and rehabilitative applications, such as a muscle relaxant to relieve pain associated with chronic clenching and grinding of teeth with TMJ Disorder. With TMJ, a BOTOX® injection is often required to relax the jaw muscles prior to surgery.
BOTOX® Cosmetic is certainly not just for women. If you are a man and think it's time to do something about those moderate to severe glabellar lines between your brows, talk to your doctor about whether BOTOX® Cosmetic is right for you.
- 6% of total BOTOX® Cosmetic procedures are performed on males (313,714 procedures). [ASPS 2008 Statistics, p.10]
- BOTOX® Cosmetic is the most popular minimally invasive physician administered aesthetic procedure for males (314,000 procedures). [ASPS 2008 Statistics]
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indication
BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in people 18 to 65 years of age for a short period of time (temporary).
IMPORTANT SAFETY INFORMATION
BOTOX® Cosmetic may cause serious side effects that can be life threatening. Call your doctor or get medical help right away if you have any of these problems any time (hours to weeks) after injection of BOTOX® Cosmetic:
- Problems swallowing, speaking, or breathing, due to weakening of associated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months
- Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including: loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice (dysphonia), trouble saying words clearly (dysarthria), loss of bladder control, trouble breathing, trouble swallowing. If this happens, do not drive a car, operate machinery, or do other dangerous activities
The dose of BOTOX® Cosmetic is not the same as, or comparable to, another botulinum toxin product.
There has not been a confirmed serious case of spread of toxin effect when BOTOX® Cosmetic has been used at the recommended dose to treat frown lines.
Serious and/or immediate allergic reactions have been reported. They include: itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint. Tell your doctor or get medical help right away if you are wheezing or have asthma symptoms, or if you become dizzy or faint.
Do not take BOTOX® Cosmetic if you: are allergic to any of the ingredients in BOTOX® Cosmetic (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as Myobloc® (rimabotulinumtoxinB), Dysport® (abobotulinumtoxinA), or Xeomin® (incobotulinumtoxinA); have a skin infection at the planned injection site.
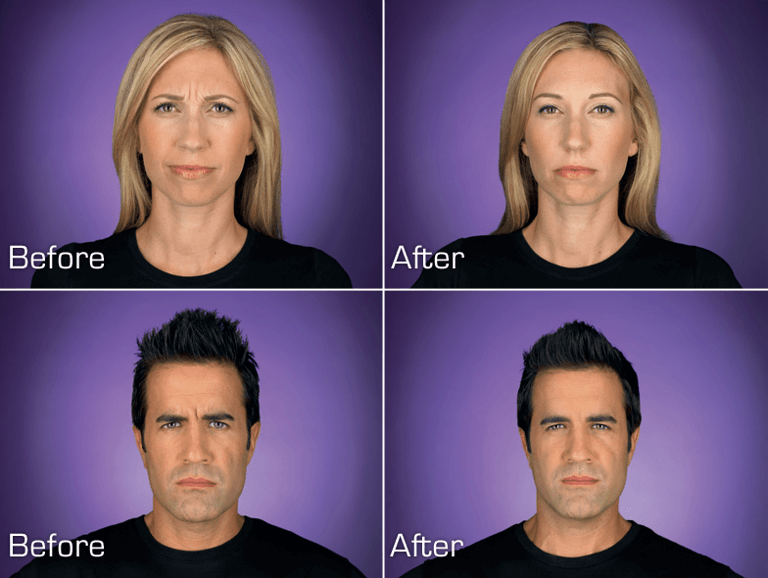
Photos of paid models taken at maximum frown before treatment with BOTOX® Cosmetic and taken at maximum frown after treatment with BOTOX® Cosmetic at day 7. Individual results may vary.
In clinical trials at day 7, 74% (299/405) of people demonstrated none or mild glabellar line severity at maximum frown as compared to 6% (8/132) in placebo; at day 30, 80% (325/405) of people demonstrated the same as compared to 3% (4/132) in placebo.1
Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/ bruising.1
1. BOTOX® Cosmetic Prescribing Information MMXII
Tell your doctor about all your muscle or nerve conditions, such as amyotrophic lateral sclerosis (ALS or Lou Gehrig's disease), myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects including severe dysphagia (difficulty swallowing) and respiratory compromise (difficulty breathing) from typical doses of BOTOX® Cosmetic.
Tell your doctor about all your medical conditions, including: plans to have surgery; had surgery on your face; weakness of forehead muscles, such as trouble raising your eyebrows; drooping eyelids; any other abnormal facial change; are pregnant or plan to become pregnant (it is not known if BOTOX® Cosmetic can harm your unborn baby); are breast-feeding or plan to breast-feed (it is not known if BOTOX® Cosmetic passes into breast milk).
Human albumin and spread of viral diseases. BOTOX® Cosmetic contains albumin, a protein component of human blood. The potential risk of spreading viral diseases [eg, Creutzfeldt-Jakob disease (CJD)] via human serum albumin is extremely rare. No cases of viral diseases or CJD have ever been reported in association with human serum albumin.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal products. Using BOTOX® Cosmetic with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received BOTOX® Cosmetic in the past.
Especially tell your doctor if you: have received any other botulinum toxin product in the last 4 months; have received injections of botulinum toxin, such as Myobloc®, Dysport®, or Xeomin® in the past (be sure your doctor knows exactly which product you received); have recently received an antibiotic by injection; take muscle relaxants; take an allergy or cold medicine; or take a sleep medicine.
Other side effects of BOTOX® Cosmetic include: dry mouth, discomfort or pain at the injection site, tiredness, headache, neck pain, and eye problems: double vision, blurred vision, decreased eyesight, drooping eyelids, swelling of your eyelids, and dry eyes.
For more information refer to the Medication Guide or talk with your doctor.
Full Product Information, including Boxed Warning and Medication Guide, has been provided to your doctor.
Photos of paid models taken at maximum frown after treatment with BOTOX® Cosmetic at month 3 and month 4. Individual results may vary.
In clinical trials at day 90, 48% (192/403) of people demonstrated none or mild glabellar line severity at maximum frown as compared to 2% (3/128) in placebo; at day 120, 25% (102/403) of people demonstrated the same as compared to 2% (2/128) in placebo.1
Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/ bruising.1
1. BOTOX® Cosmetic Prescribing Information MMXII
Dermal Fillers
Dermal fillers are injectable substances used to restore volume, contour, and smooth out wrinkles and fine lines on the face. They are typically made from substances such as hyaluronic acid, which is a naturally occurring substance in the body, or synthetic materials.
Dermal fillers can be used to enhance facial features such as the lips, cheeks, and chin, and they can also be used to treat nasolabial folds, marionette lines, and other signs of aging. The results are typically temporary and can last anywhere from several months to a year or more, depending on the type of filler used and the area being treated.
Schedule an appointment today!